Faulty genes fixed before birth
A new study shows that a biomedical tool can successfully deliver genetic material to fix faulty genes in developing fetal brain cells. Tested in mice, this technology may help prevent genetic-based neurodevelopmental conditions like Angelman syndrome and Rett syndrome before birth.
Credit: UC Davis
“This tool could revolutionize treatments for neurodevelopmental disorders by correcting genetic issues during critical stages of brain development,” said lead author Aijun Wang, a UC Davis professor.
The study, published in ACS Nano, is a collaboration between UC Davis and UC Berkeley. The goal is to use this technology to treat genetic conditions diagnosed through prenatal testing, preventing damage as cells mature.
Innovative mRNA Delivery
In certain genetic conditions, the body produces too much or too little of certain proteins, causing problems. Delivering proteins directly to cells is challenging, so researchers found a way to deliver mRNA instead, which the cells use to make the needed proteins. This is done using lipid nanoparticles (LNPs) to carry the mRNA into cells.
This technique has already been used in COVID-19 vaccines, and the team has developed a new, safer LNP to improve efficiency. Once inside the cell, the LNP breaks down, releasing the mRNA so it can be translated into functional proteins.
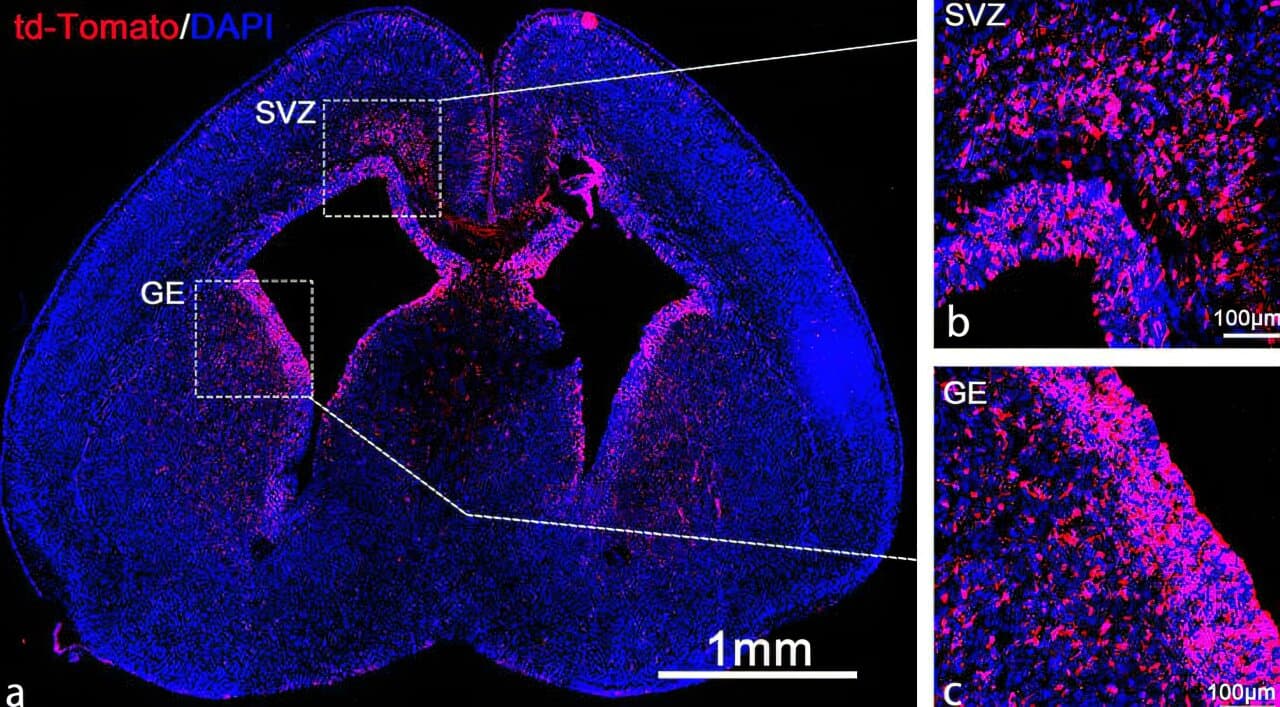
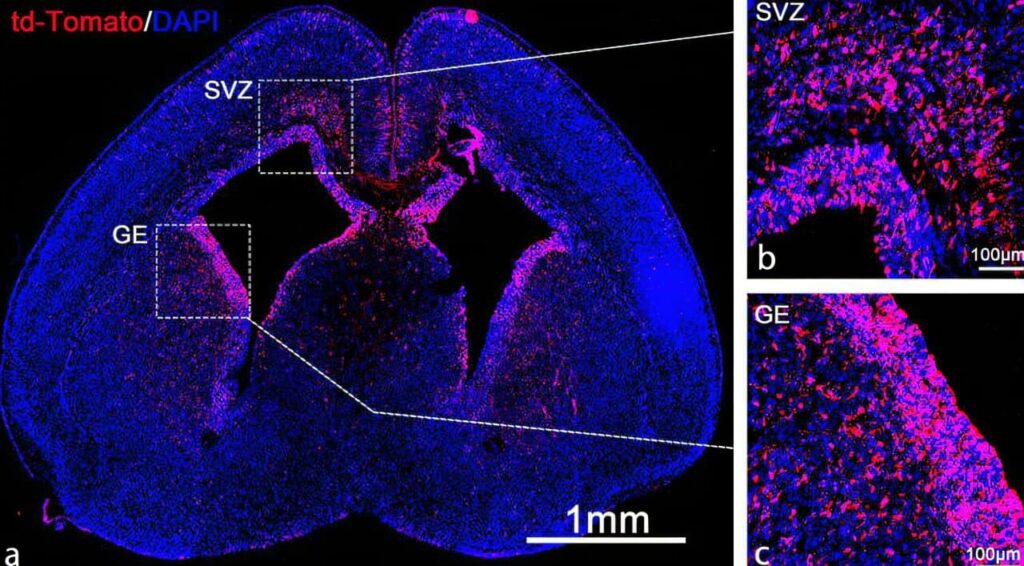
The researchers tested this method by delivering mRNA for the Cas9 protein, which is used in gene editing, to the brains of fetal mice. The Cas9 protein edits faulty genes responsible for conditions like Angelman syndrome. By delivering this treatment early, before the blood-brain barrier is fully formed, the disease progression could be stopped in utero.
Promising Results
The study showed that the LNP system efficiently delivered mRNA, leading to gene edits in 30% of brain stem cells. These cells spread throughout the developing brain, and more than 60% of neurons in the hippocampus and 40% in the cortex were affected.
This approach could significantly reduce or eliminate symptoms in newborns with genetic brain disorders, and Wang believes the results may be even better in diseased models.