Efficient Leap: Nickel-Powered Alkaline Electrolyzer Rivals PEM in Green Hydrogen Production
Researchers from the Technical University of Berlin, Helmholtz-Zentrum Berlin (HZB), the University of Freiburg’s Department of Microsystems Engineering (IMTEK), and Siemens Energy have made a significant breakthrough in the development of a highly efficient alkaline membrane electrolyzer (AEM). This innovative technology approaches the performance of established proton-exchange membrane (PEM) electrolyzers, but with a crucial difference: it utilizes inexpensive nickel compounds as the anode catalyst, replacing the costly and rare metal iridium.
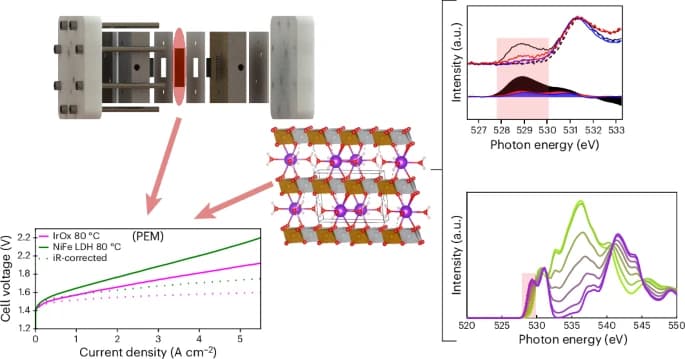
Image Credits: Nature Catalysis
The achievement is noteworthy as it brings the production of green hydrogen a step closer to being both sustainable and cost-effective. Hydrogen is poised to play a major role in the future energy system, serving as an energy storage medium, a clean fuel, and a valuable raw material for the chemical industry. When produced through water electrolysis using electricity from solar or wind power, hydrogen can be virtually climate-neutral.
The research team’s AEM electrolyzer combines the advantages of both PEM and classic liquid alkaline electrolysis systems, without requiring rare precious metals like iridium. To develop this technology, the team employed a novel coating process to directly apply nickel double hydroxide compounds (enhanced with iron, cobalt, or manganese) onto an alkaline ion exchange membrane. This process, tested in prototype cells built in Freiburg, demonstrates excellent scalability.
Detailed insights into the molecular processes during electrolysis were gained through operando measurements conducted at the Berlin X-ray source BESSY II. A collaborative effort with theory teams from the U.S. and Singapore provided a comprehensive molecular description of the catalytic processes. Notably, the research revealed that the high efficiency of the nickel-based catalyst stems from a phase transition to a highly active gamma phase, exhibiting similarities to iridium’s catalytic mechanism while also displaying unique molecular differences.
Published in the journal Nature Catalysis, this study significantly advances our understanding of the fundamental catalysis mechanisms of nickel-based electrode materials. The breakthrough paves the way for further industrial evaluation, showcasing the potential of AEM water electrolyzers to efficiently produce green hydrogen, thereby supporting the transition to a more sustainable energy economy.